Konruns(603590)
Search documents
康辰药业(603590) - 康辰药业第四届监事会第十六次会议决议公告
2025-10-15 13:15
北京康辰药业股份有限公司(以下简称"公司")第四届监事会第十六次会 议于 2025 年 10 月 15 日以电子邮件方式通知全体监事,本次会议以紧急会议的 形式召集与召开,本次监事会的通知时限已经公司全体监事一致同意豁免,会议 于 2025 年 10 月 15 日以传签方式形成有效决议。本次会议召集人为监事会主席 邸云女士,会议应当参加表决的监事 3 名,实际参加表决的监事 3 名。本次会议 的参与表决人数及召开程序符合《公司法》和《公司章程》的有关规定,合法有 效。 证券代码:603590 证券简称:康辰药业 公告编号:临 2025-061 北京康辰药业股份有限公司 第四届监事会第十六次会议决议公告 本公司监事会及全体监事保证本公告内容不存在任何虚假记载、误导性陈述 或者重大遗漏,并对其内容的真实性、准确性和完整性承担法律责任。 一、监事会会议召开情况 二、监事会会议审议情况 1、审议通过《关于以集中竞价交易方式回购公司股份方案的议案》 具体内容详见公司同日于指定信息披露媒体披露的《关于以集中竞价交易方 式回购股份的回购报告书》。 表决结果:3 票同意、0 票反对、0 票弃权。 特此公告。 北京康辰药业股份 ...
康辰药业(603590) - 康辰药业第四届董事会第二十次会议决议公告
2025-10-15 13:15
2025年10月16日 一、董事会会议召开情况 北京康辰药业股份有限公司(以下简称"公司")第四届董事会第二十次会 议于 2025 年 10 月 15 日以电子邮件方式通知全体董事,本次会议以紧急会议的 形式召集与召开,本次董事会的通知时限已经公司全体董事一致同意豁免,会议 于 2025 年 10 月 15 日以传签方式形成有效决议。本次会议召集人为董事长刘建 华先生,会议应当参加表决的董事 9 名,实际参加表决的董事 9 名。本次会议的 参与表决人数及召开程序符合《公司法》和《公司章程》的有关规定,合法有效。 二、董事会会议审议情况 1、审议通过《关于以集中竞价交易方式回购公司股份方案的议案》 具体内容详见公司同日于指定信息披露媒体披露的《关于以集中竞价交易方 式回购股份的回购报告书》。 表决结果:9 票同意、0 票反对、0 票弃权。 特此公告。 北京康辰药业股份有限公司董事会 证券代码:603590 证券简称:康辰药业 公告编号:临 2025-060 北京康辰药业股份有限公司 第四届董事会第二十次会议决议公告 本公司董事会及全体董事保证本公告内容不存在任何虚假记载、误导性陈述或 者重大遗漏,并对其内容的真 ...
康辰药业:拟以5000万元-1亿元回购公司股份
Xin Lang Cai Jing· 2025-10-15 13:08
康辰药业公告,拟以5000万元-1亿元回购公司股份,回购价格不超过82元/股。本次回购股份全部用于 员工持股计划或股权激励。预计回购股份数量为60.98万股-121.95万股,占公司总股本的0.38%-0.77%。 回购资金来源为公司自有资金或自筹资金,回购期限为董事会审议通过后12个月内。 ...
生物制品板块10月15日涨1.78%,赛升药业领涨,主力资金净流入3.31亿元
Zheng Xing Xing Ye Ri Bao· 2025-10-15 08:37
Core Insights - The biopharmaceutical sector experienced a rise of 1.78% on October 15, with Sai Sheng Pharmaceutical leading the gains [1] - The Shanghai Composite Index closed at 3912.21, up 1.22%, while the Shenzhen Component Index closed at 13118.75, up 1.73% [1] Company Performance - Sai Sheng Pharmaceutical (300485) closed at 12.28, with a gain of 9.16% and a trading volume of 369,300 shares [1] - Kanghua Biological (300841) closed at 77.71, up 6.02%, with a trading volume of 49,400 shares [1] - Kangchen Pharmaceutical (603590) closed at 49.11, increasing by 5.84%, with a trading volume of 40,700 shares [1] - San Sheng Guo Jian (688336) closed at 53.00, up 5.68%, with a trading volume of 56,800 shares [1] - Bai Pu Sai Si (301080) closed at 57.73, gaining 5.23%, with a trading volume of 28,000 shares [1] - Rongchang Biological (688331) closed at 96.05, up 5.20%, with a trading volume of 72,700 shares [1] - Shenzhou Cell (688520) closed at 55.03, increasing by 4.90%, with a trading volume of 42,700 shares [1] - Kang Le Wei Shi (920575) closed at 12.94, up 4.61%, with a trading volume of 53,900 shares [1] - Tonghua Dongbao (600867) closed at 8.96, gaining 4.55%, with a trading volume of 543,800 shares [1] - Olin Biological (61688919) closed at 24.96, up 4.22%, with a trading volume of 65,800 shares [1] Market Capital Flow - The biopharmaceutical sector saw a net inflow of 331 million yuan from institutional investors, while retail investors experienced a net outflow of 234 million yuan [2] - The main funds showed a net inflow in several companies, including Changchun High-tech (000661) with 66.94 million yuan and Tonghua Dongbao (600867) with 62.56 million yuan [3] - Retail investors showed significant outflows in companies like Sai Sheng Pharmaceutical (300485) with 50.24 million yuan and Rongchang Biological (688331) with 38.34 million yuan [3]
康辰药业股价跌5.07%,信达澳亚基金旗下1只基金重仓,持有73.06万股浮亏损失184.11万元
Xin Lang Cai Jing· 2025-10-14 03:16
Group 1 - The core point of the news is that Kangchen Pharmaceutical's stock has experienced a decline of 5.07% on October 14, with a cumulative drop of 7.7% over three consecutive days, leading to a market capitalization of 7.512 billion yuan [1] - Kangchen Pharmaceutical, established on September 3, 2003, and listed on August 27, 2018, focuses on innovative drug research and development, with its main business revenue composition being 70.77% from Suling, 29.15% from Salmon Calcitonin, and 0.09% from other sources [1] Group 2 - According to data, the Xinda Aoya Fund holds Kangchen Pharmaceutical as its tenth largest position, with 730,600 shares representing 3.38% of the fund's net value, resulting in a floating loss of approximately 1.8411 million yuan today and a total floating loss of 3.0247 million yuan during the three-day decline [2] - The Xinda Health China Mixed A Fund (003291) was established on August 18, 2017, with a current scale of 534 million yuan, achieving a year-to-date return of 26.2% and a one-year return of 24.88% [2]
康辰药业跌2.09%,成交额4316.83万元,主力资金净流出298.21万元
Xin Lang Cai Jing· 2025-10-14 02:15
Core Viewpoint - 康辰药业's stock has experienced fluctuations, with a year-to-date increase of 112.12% but a recent decline of 9.00% over the last five trading days [1] Group 1: Stock Performance - As of October 14, 康辰药业's stock price was 48.62 yuan per share, with a market capitalization of 7.748 billion yuan [1] - The stock has seen a trading volume of 43.1683 million yuan and a turnover rate of 0.55% [1] - The stock has been on the龙虎榜 four times this year, with the most recent appearance on September 1 [1] Group 2: Financial Performance - For the first half of 2025, 康辰药业 reported a revenue of 461 million yuan, representing a year-on-year growth of 13.79% [2] - The net profit attributable to shareholders for the same period was 91.046 million yuan, reflecting a year-on-year increase of 14.95% [2] Group 3: Shareholder Information - As of June 30, 2025, 康辰药业 had 9,970 shareholders, a decrease of 10.82% from the previous period [2] - The average number of circulating shares per shareholder increased by 12.14% to 15,771 shares [2] - The company has distributed a total of 437 million yuan in dividends since its A-share listing, with 175 million yuan distributed over the last three years [3] Group 4: Institutional Holdings - As of June 30, 2025, notable institutional shareholders include 鹏华医药科技股票A and 易方达医疗保健行业混合A, with new entries in the top ten shareholders [3]
康辰药业今日大宗交易折价成交31万股,成交额1411.12万元
Xin Lang Cai Jing· 2025-10-13 09:46
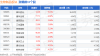
| 交易日期 | 证券简称 | 证券代码 | 成交价(元) 成交金额(万元) 成交量( *) 买入营业部 | | | | 卖出营业部 | 是否为专场 | | --- | --- | --- | --- | --- | --- | --- | --- | --- | | 025-10-13 | 康辰药业 | 603590 | 45.52 31 | 1411.12 | 貓影響解 | 親觀體 | | ya | 10月13日,康辰药业大宗交易成交31万股,成交额1411.12万元,占当日总成交额的8.11%,成交价 45.52元,较市场收盘价49.66元折价8.34%。 ...
康辰药业(603590) - 康辰药业关于召开2025年半年度业绩说明会的公告
2025-10-13 08:30
证券代码:603590 证券简称:康辰药业 公告编号:临 2025-059 北京康辰药业股份有限公司 关于召开 2025 年半年度业绩说明会的公告 本公司董事会及全体董事保证本公告内容不存在任何虚假记载、误导性陈述 或者重大遗漏,并对其内容的真实性、准确性和完整性承担法律责任。 北京康辰药业股份有限公司(以下简称"公司")已于 2025 年 8 月 28 日发布公司 2025 年半年度报告,为便于广大投资者更全面深入地了解公司 2025 年半年度经营成果、财务状况,公司计划于 2025 年 10 月 21 日下午 16:00-17:00 举行 2025 年半年度业绩说明会,就投资者关心的问题进行交流。 一、 说明会类型 本次投资者说明会以网络互动形式召开,公司将针对 2025 年半年度经营 成果及财务指标的具体情况与投资者进行互动交流和沟通,在信息披露允许 的范围内就投资者普遍关注的问题进行回答。 二、 说明会召开的时间、地点 (一) 会议召开时间:2025 年 10 月 21 日 下午 16:00-17:00 (二) 会议召开地点:上证路演中心 会议召开时间:2025 年 10 月 21 日(星期二) 下午 ...
北京康辰药业股份有限公司 关于控股股东部分股份解除质押的公告
Zhong Guo Zheng Quan Bao - Zhong Zheng Wang· 2025-10-11 04:58
一、股东本次股份解除质押情况 ■ 二、股东累计质押股份情况 截至本公告披露日,刘建华先生及其一致行动人累计质押股份情况如下: ■ 登录新浪财经APP 搜索【信披】查看更多考评等级 本公司董事会及全体董事保证本公告内容不存在任何虚假记载、误导性陈述或者重大遗漏,并对其内容 的真实性、准确性和完整性承担法律责任。 重要内容提示: ●截至本公告披露日,北京康辰药业股份有限公司(以下简称"公司")控股股东刘建华先生持有公司股 份数量为49,827,760股,占公司总股本比例为31.27%;本次解除股份质押后,刘建华先生累计质押公司 股份数量为4,646,840股,占其持股数量比例为9.33%。 近日,公司获悉刘建华先生将其质押给中国银河证券股份有限公司的420万股无限售条件流通股办理了 解除质押,具体相关情况如下: 公司将持续关注股东所持公司股份的质押情况,严格遵守相关规定,及时履行信息披露义务。 特此公告。 2025年10月11日 北京康辰药业股份有限公司董事会 ...
北京康辰药业股份有限公司关于控股股东部分股份解除质押的公告
Shang Hai Zheng Quan Bao· 2025-10-10 19:42
登录新浪财经APP 搜索【信披】查看更多考评等级 证券代码:603590 证券简称:康辰药业 公告编号:临2025-058 北京康辰药业股份有限公司关于控股股东部分股份解除质押的公告 本公司董事会及全体董事保证本公告内容不存在任何虚假记载、误导性陈述或者重大遗漏,并对其内容 的真实性、准确性和完整性承担法律责任。 重要内容提示: ● 截至本公告披露日,北京康辰药业股份有限公司(以下简称"公司")控股股东刘建华先生持有公司股 份数量为49,827,760股,占公司总股本比例为31.27%;本次解除股份质押后,刘建华先生累计质押公司 股份数量为4,646,840股,占其持股数量比例为9.33%。 近日,公司获悉刘建华先生将其质押给中国银河证券股份有限公司的420万股无限售条件流通股办理了 解除质押,具体相关情况如下: 一、股东本次股份解除质押情况 公司将持续关注股东所持公司股份的质押情况,严格遵守相关规定,及时履行信息披露义务。 特此公告。 北京康辰药业股份有限公司董事会 2025年10月11日 ■ 二、股东累计质押股份情况 截至本公告披露日,刘建华先生及其一致行动人累计质押股份情况如下: ■ ...